Written By Keshav Mohta (Grade 11)
One of the most fundamental physical needs for human survival is a reliable supply of oxygen (O2). Long-term space exploration is impossible without a self-sustaining supply of this vital gas. There are numerous proposed solutions for oxygen generation. Some have been tested and others are still just ideas.
Electrolysis of Water
Electrolysis of water is the most common method for generating oxygen and is currently being used aboard the ISS.
Electrodes are placed in ionized water and a potential difference is created to allow an electric current to flow between them. The current flows through the water as well ionizing it into H+ and OH– ions.
The electrodes in the water are charged and the OH– anions are attracted to the anode. The H+ cations move to the cathode. The H+ ions receive electrons to form H2 gas which bubbles up near the cathode. Meanwhile OH– loses electrons to form O2 gas, which also bubbles out. The hydrogen and oxygen gases can be collected separately.
While this is the simplest and most viable method, it is highly inefficient in its use of water, a precious resource which may not be found in abundance in outer space. To improve water efficiency, we can additionally use the Sabatier Reaction which reacts the hydrogen produced in electrolysis with carbon dioxide from Mars’ atmosphere (~98% in composition) to produce methane and water. The water can be recycled while methane can be used as a fuel.
MOXIE
MOXIE stands for Mars Oxygen In-Situ Resource Utilization Experiment. It is a device created by NASA which electrochemically splits carbon dioxide into oxygen by releasing a by-product of carbon monoxide. It was recently tested from 2021 to 2023 on the Perseverance rover sent to Mars.
One of the main disadvantages of MOXIE is that it releases carbon monoxide. Carbon monoxide is very harmful for humans. If by any chance the gas leaks into the habitation chamber some of the astronauts may die. Additionally, it requires a temperature of 800 oC, which requires a lot of energy to maintain.
Cyanobacteria
Cyanobacteria, also called blue-green algae, are photosynthetic bacteria. They normally grow in water and can survive in dimly lit areas. However, they require a constant temperature of 20-30 oC.
Cyanobacteria can be used to create oxygen through the process of photosynthesis. Cyanobacteria are said to have been the main creators of oxygen in Earth’s atmosphere. They have also been found in the deep trenches of the ocean, Antarctica as well as on the edge of the ISS. A Mars mission could carry a small amount of cyanobacteria with itself. The bacteria require carbon dioxide, sunlight, and water and can double in 30 minutes. This would help create a large amount of cyanobacteria on Mars, which would provide a constant flow of oxygen by utilising the abundant carbon dioxide in the Martian air.
Artificial Photosynthesis
Another method to convert carbon dioxide to oxygen is by Artificial Photosynthesis. Photosynthesis, as we all have learned in school, requires carbon dioxide, sunlight, water and chlorophyll. Sunlight and carbon dioxide are already available on Mars and chlorophyll is easy to extract from leaves.
There is immense research happening across the world on artificial silk leaves. These artificial leaves carry out the process of photosynthesis clinically. They use semiconductor materials and capture sunlight to do photosynthesis.
Ultraviolet Lasers
Ultraviolet light has a wavelength less than that of violet colour. Its wavelength is shorter than 400 nanometres. The shortest wavelength of ultraviolet light is called vacuum ultraviolet light. Using vacuum ultraviolet light, it is possible to split carbon dioxide to get oxygen and carbon. However, breaking a carbon dioxide molecule requires a lot of energy and generating vacuum ultraviolet light is extremely difficult.
If the ultraviolet laser can strike the carbon dioxide exactly on the carbon atom, the atom will be pushed outwards and the oxygen will bond while the carbon atom will bond with another carbon atom from another carbon molecule. However, if the laser strikes in a different place it will result in the carbon dioxide molecule breaking up into carbon monoxide and oxygen. This process can be seen in the picture below.
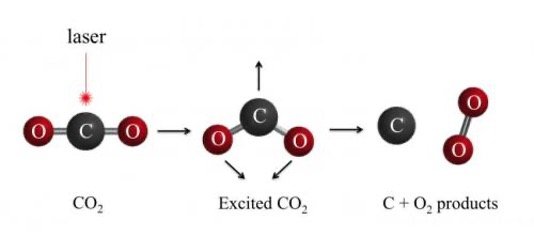
In conclusion, each of the methods have their benefits and challenges. All these methods are currently being explored by researchers to see how implementable they are for the future.
Featured Image Courtesy – New Scientist